Product
All categories

Infrared composting method
Infrared composting method (Reference GB/T 19277.1-2011 Determination of the final aerobic biodegradation capacity of materials under controlled compost conditions - Method for determination of released carbon dioxide - Part 1: General method) :
Experimental purpose
1. Meet the testing requirements of GB/T19277.1-2011 standard;
2. Meet the testing requirements of ISO14855.1 standard;
3. For the study of biodegradability of bio-based materials and products;
4. For composting biodegradability detection;
5. Standard experiment for measuring carbon dioxide release under other simulated aerobic compost conditions;
Two experimental principles
This experiment system simulates the process of biodegradation of bio-based materials under aerobic composting conditions. In the aerobic environment, microorganisms consume oxygen and produce carbon dioxide, water and other inorganic substances with bio-based materials as the carbon source. The generated carbon dioxide is monitored in real time by high-precision infrared sensors, and the real-time concentration of detected carbon dioxide is integrated with the flow rate and time to calculate the carbon dioxide release from the biodegradation of the sample. The biodegradation rate is the percentage of the carbon dioxide released by the sample biodegradation and its theoretical carbon dioxide release.
Three experimental materials
1. Distilled water
2. Decomposed compost (3-4 months of fertilizer age, provided by Tomorgan Biology)
3. Thin layer chromatographic grade (TLC) cellulose
4. Sodium lime
5. Color-changing silicone
6. Urea
Four experimental steps
1. Instrument preparation:
1) Place the decarbonization bottle, reaction bottle, condensation bottle and dehumidification bottle in the corresponding position, and connect the hose.
2) Open the instrument power supply, open the ventilation system, infrared detection system, temperature control system; Check whether the air tightness of the reaction system is normal; Check whether the infrared detection data is within the normal range; Check that the temperature is maintained at a constant temperature.
2. Preparation of inoculum:
1) Weigh Morgan's homemade compost, add water to activate it for 24 hours, set aside.
3. Experimental materials added:
1) Add sodium lime to decarbonized bottle.
2) Add the color-changing silicone to the dehumidifier bottle.
4. Sample preparation:
1) The bulk experimental material is processed into a uniform shape with a surface area of no more than 2cm*2cm, and 3 parts are weighed, each of which is 30g.
2) Weigh 3 portions of fiber, 30g each.
5. Sample addition:
1) Weigh 300g of activated compost, add the cellulose and the tested matter to the compost, mix them, and put them into the reaction bottle.
2) Connect the reaction system and check the tightness of the reaction system again.
6, adjust the instrument parameters:
1) Adjust the flow rate of each channel to 150ml/min.
2) Clear the accumulated data of each channel and start the experimental cumulative data.
7. Start of experiment:
1) After the start of the experiment, check whether the growth state of the experimental data meets the expectation and whether the instrument parameter Settings are normal every day.
5. M9000 degradation experiment record report
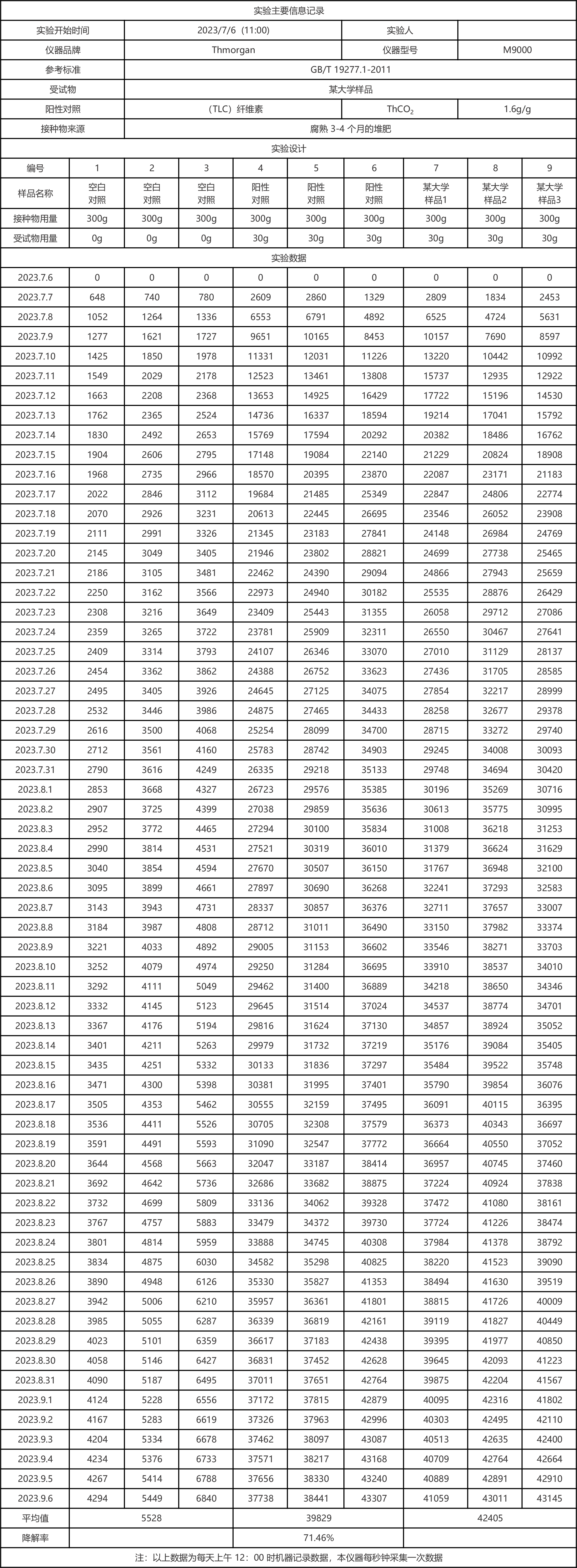
Six Carbon dioxide release curve:
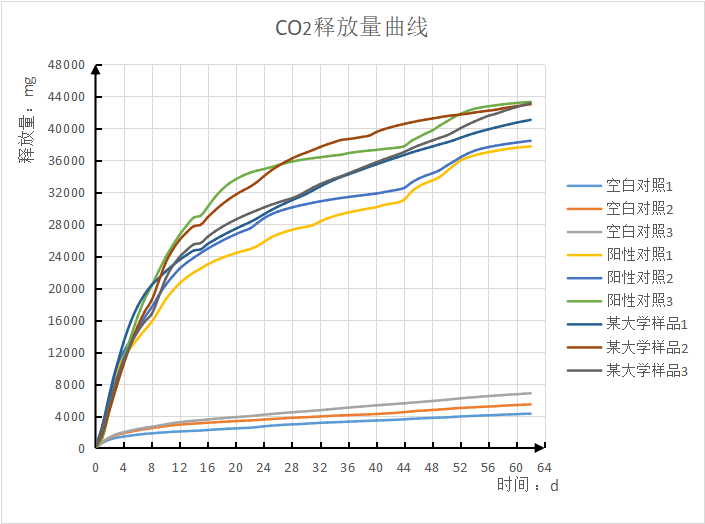
Seven Analysis of experimental results:
1. The experiment started on July 6, 2023, and has been conducted for 62 days so far.
2. Blank control data: Bottle No. 1, 2 and 3 was the blank control group, releasing 5528mg of carbon dioxide.
3. Positive control data: Bottles No.4, 5 and 6 were the positive control group, releasing 34301mg (39829-5528) of carbon dioxide, and the degradation rate reached 71.46% (34301/(1.6*30000)*100%).
4, test sample data: 7, 8, 9 bottle is a university sample, released 36877mg of carbon dioxide (42405-5528).
5. Overall data analysis: the parallelism of the experimental group was good, and the relative deviation of biodecomposition percentage in GB/T 19277.1 was less than 20%; The degradation rate of the control group and the experimental group tended to be stable.