GB/T19276.1
GB/T19276.1
First, the purpose of the experiment
Determine the final aerobic biofraction of a sample under aqueous culture conditions.
Second, the experimental principle
Aerobic microorganisms are used in aqueous systems to determine the biodecomposition rate of experimental materials.
At 25 °C, oxygen consumption was determined by coulombic method by electrolytic oxygen supply, closed reaction, and used to determine the biochemical oxygen demand of the sample.
The percentage of actual biological oxygen demand to theoretical oxygen demand is the rate of biodegradation.
3. Experimental materials
1. Distilled water
2. Homemade rotted compost (3-4 months fat age)
3. Cellulose
4. Alkali lime
5. Anhydrous copper sulfate
6. Inorganic salt solution A fixed volume to 1000ml:
KH2PO4 (anhydrous) 8.5g;
K2HPO 4 (anhydrous) 21.75g;
Na2HPO4·2H2O 33.4g;
NH4CI :0.5g
7. Inorganic salt solution B: dissolve MgSO4·2H2O 22.5g in water, fixed volume to 1000ml;
8. Inorganic salt solution C: dissolve CaCI2·2H2O 36.4g in water, fixed volume to 1000ml;
9. Inorganic salt solution D: dissolve FeCI3·6H2O 0.25g in water, fixed volume to 1000ml;
10, solution E: 3 ml solution A, 0.3 ml solution B, 0.3 ml solution C, 0.3 ml solution D, constant volume 300 ml;
4. Experimental instruments
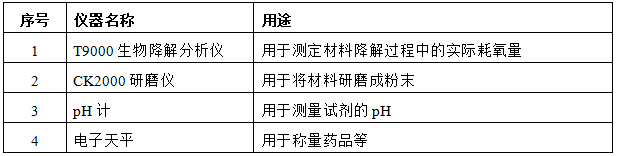
5. Experimental steps
1. Preparation of inoculums
Add 100g of unsterilized compost per 10ml of solution E, mix well with shaking, then stand for 30min, filter the suspension with coarse porous filter paper, and pour the filtrate into the reaction flask to obtain an inoculum with a strain concentration (V/V) of 1%-5%.
2. Preparation of experimental materials and reference materials
2.1 Weigh an appropriate amount of sample and freeze-grind the sample into powder with a grinder for experiments.
2.2 Use aniline, microcrystalline cellulose powders or well-defined biodegradable polymers as positive control reference materials.
3. Start the experiment
3.1 Put the air cylinder, electrolytic bottle and reaction bottle into the corresponding card slot;
3.2 Turn on the power, set 25°C, open the internal circulation, and start heating;
3.3 T9000 air balance experiment for 4 hours;
3.4 T9000 no-load air leakage test for 12 hours;
3.5 Open the reaction bottle stopper after the no-load air leakage test;
3.6 Put the inoculum and materials into reaction bottles according to the following table and mix well;
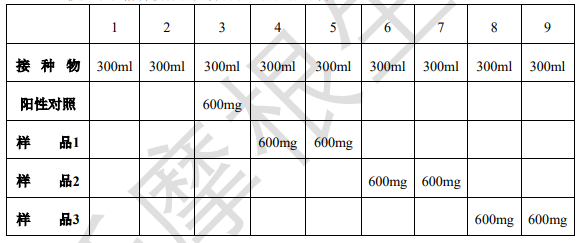
Note: The volume of the reaction bottle is 500ml; Measure the pH inside the bottle and adjust it to pH=7.
3.7 Add alkali lime to the glass tray and fix the tray to the inner cap of the experimental vial;
3.8 Insert the No. 1-9 reaction cork into the reaction bottle and screw the reaction bottle cap tightly to prevent air leakage;
3.9 Open the U-tube knob (so that the air pressure at both ends of the U-tube is consistent with atmospheric pressure), equilibrate for 4 hours;
3.10 Close the U-tube knob (the reaction system is closed with the atmosphere, but the air pressure at both ends of the upper and lower U-tube is the same), equilibrate for 2h;
3.11 Close the U-tube knob (both ends of the U-tube are closed) and start the experiment;
4. End the experiment
The maximum experimental period is 6 months.
End the experiment when the biochemical oxygen demand reaches a stable phase and no further biodecomposition is expected.
At the end of the test, the concentration of nitrate and nitrite in the reaction flask is determined immediately, or the appropriate sample is taken and stored for measurement. These values are used to correct for deviations in the degree of biodecomposition due to nitrification.
5. Validity of results
A test can only be considered valid if it meets the following criteria:
5.1 At the end of the test, the percentage of biodecomposition of the reference material > 60%;
5.2 The relative deviation between the biodegradable percentages of each compost container at the end of the test does not exceed 20%;
5.3 At the end of the experiment, the BOD of the blank group did not exceed the upper limit of empirical values.
6. Calculation of results
1. Calculate theoretical oxygen consumption
A compound with a relative molecular mass of Mr, CcHhClClN nS PpNaNa Oo, if its chemical composition is known or can be measured by elemental analysis, ThOD can be calculated using the following formula:
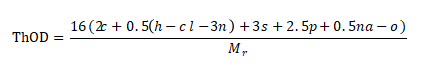
This calculation assumes that carbon is converted to carbon dioxide, hydrogen to water, phosphorus to phosphorus pentoxide, sulfur to positive hexavalent oxidation, and halogens are removed as hydrogen halide. The oxides of nitrogen, phosphorus and sulfur must be analyzed and confirmed, and this calculation also assumes that nitrogen becomes nitrate and nitrite.
2. Calculate the actual oxygen consumption per unit experimental material
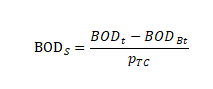
In the formula:
BODS represents the BOD value per unit of experimental material, expressed in milligrams per gram of experimental material, in milligrams per gram (mg/g)
BOD t represents the BOD value of the vial containing the experimental material at timet in milligrams per liter (mg/L)
BODBt represents the BOD value of the blank vial at time t in milligrams per liter (mg/L)
PTC indicates the concentration of experimental material in the reaction flask in milligrams per liter (mg/L)
Calculate the percentage of biodecomposition (Dt) as follows:
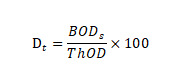
If the relative deviation of each outcome is less than 20%, the average biodecomposition percentage is calculated, otherwise, the values of each reaction flask are used separately.
The percentage of biodecomposition of the reference material is calculated in the same way.