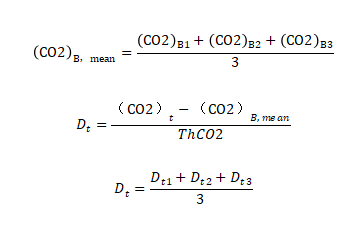GB/T19277.2
GB/T19277.2
First, the purpose of the experiment
Under controlled composting conditions, its final aerobic biodegradability is determined by measuring the amount of carbon dioxide emitted.
Second, the experimental principle
Under the action of microorganisms, bio-based materials consume oxygen to decompose to produce carbon dioxide, water and other inorganic substances, and the carbon dioxide produced is absorbed by the absorption tower, and the amount of carbon dioxide released by the reaction tower is measured by the change in the quality of the carbon dioxide absorption device. The percentage of the actual CO2 release of the sample to the theoretical CO2 release of the sample is the biodegradation rate.
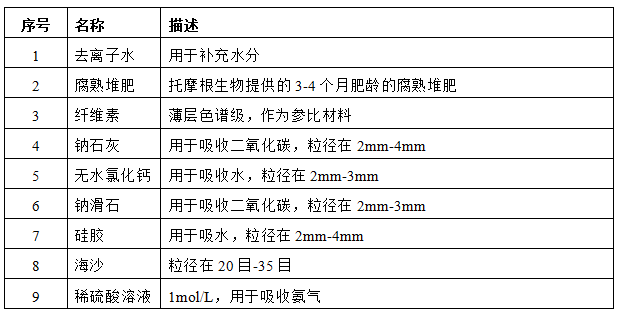
3. Experimental materials
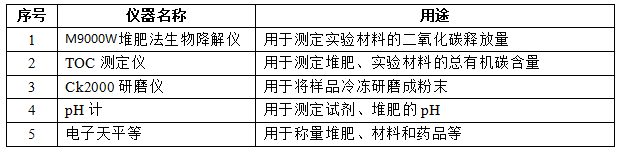
4. Experimental instruments
5. Experimental steps
1. Preparation of compost
Weigh a sufficient amount of compost produced by Tomergen Bio, compost age is 3-4 months of rotten compost, humidity is 55%-60%, pH is between 7.0-9.0.
2. Prepare sea sand
Soak the sea sand in clean water. Floating impurities are removed by precipitation, the sea sand is fully rinsed, the water is drained and dried at about 105°C.
Add appropriate deionized water to adjust the moisture humidity to about 15%.
3. Prepare experimental materials and reference materials
The experimental materials are frozen into powder with liquid nitrogen in a grinder, and the particle size of the largest particles cannot exceed 250 μm.
Thin layer chromatography grade (TLC) cellulose was used as the positively controlled reference material with a particle size of less than 20 μm.
4. Start the experiment
3.1 Add sufficient amount of soda lime to the decarburization column;
3.2 Add an appropriate amount of deionized water to the saturated steam generating bottle;
3.3 Add an appropriate amount of dilute sulfuric acid solution to the ammonia removal bottle;
3.4 Add sufficient amount of silica gel and sewage calcium chloride to the dehumidification tower;
3.5 Add sufficient amounts of soda lime and sodium talc to the absorption tower;
3.6 Add the corresponding substance to the reaction flask as shown in the table below.
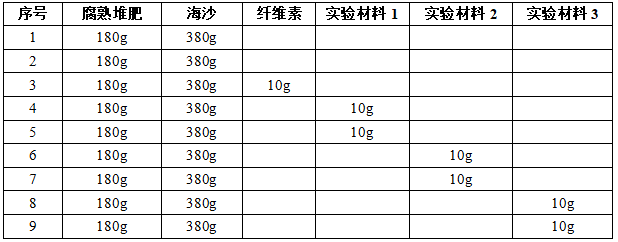
3.7 Turn on the power supply of M9000W, set the temperature to 58°C, and start heating;
3.8 Turn on oxygen ventilation, adjust the gas flow meter, and set the gas flow rate at 100-200ml/L;
3.9 Take the absorption tower regularly, weigh the absorption tower, and record the change of the weight of the absorption tower in detail;
4. End the experiment
The maximum experimental period is 6 months.
The experiment ends when the carbon dioxide release reaches a plateau phase and no further biodecomposition is expected.
5. Validity of results
5.1 At the end of the test, the percentage of biodecomposition of the reference material exceeded 60%;
5.2 At the end of the experiment, the relative deviation between the biodecomposition percentages of each reaction system in each group did not exceed 20%;
5.3 Inoculum in blank containers yields 10 mg CO50/g volatile solids (average) to 2 mg CO150/g volatile solids (average) 2 days prior to the experiment.
6. Calculation of results
1. Calculate the theoretical release of carbon dioxide
The theoretical carbon dioxide release (ThCO2) of the experimental material is calculated according to the following formula, expressed as (g):
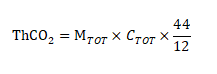
In the formula:
MTOT represents the total dry solid of the experimental material added to the reaction flask at the beginning of the experiment in units (g)
CTOT represents the ratio of total organic carbon to total dry solids in the experimental material in grams per gram (g/g)
44 and 12 indicate the molecular weight of carbon dioxide and the atomic weight of carbon, respectively
2. Calculate the percentage of biological decomposition
The biodecomposition percentage DT(%) of the experimental material was calculated according to the cumulative amount of carbon dioxide released in each test cycle.
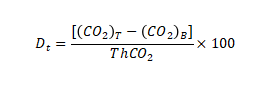
In the formula:
(CO2)T represents the cumulative amount of carbon dioxide released per vial containing the experimental mixture in grams per container (g/container)
(CO2)T represents the average of the cumulative amount of carbon dioxide released in blank containers in grams per container (g/container)
ThCO2 represents the theoretical amount of carbon dioxide produced by the experimental material in grams per container (g/container)
If the relative deviation of each outcome is less than 20%, the average biodecomposition percentage is calculated, otherwise, the values of each reaction flask are used separately.
The biodecomposition rate of the reference material was calculated using the same method.
7. Experimental report
Material degradation experiment report
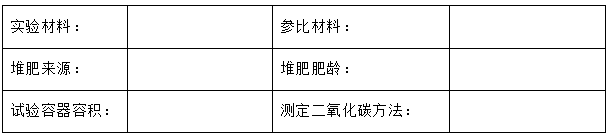
Experimental results:
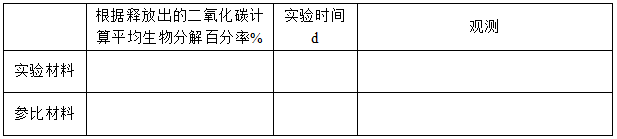
Validity judgment basis:
Is the percentage of biodecomposition of the reference material > 45% after 70 days?
□Yes □No
Is the relative deviation in the percentage of biodecomposition of the reference material in different containers < 20% at the end of the test?
□Yes □No
Is the average amount of CO10 produced by the blank container within 50 days before the test between 2 mg CO150/g volatile solids and 2 mg CO<>/g volatile solids?
□Yes □No
Percentage of biodecomposition calculated based on the amount of carbon dioxide released
Experimental material or reference material: TOC: g/g ThCO2: g/container
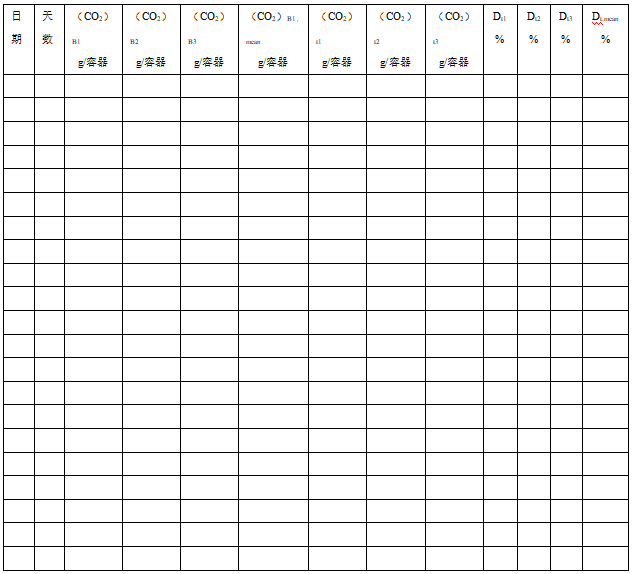
(CO2)B – the cumulative amount of carbon dioxide produced by the measured blank test;
(CO2)t – the cumulative amount of carbon dioxide produced by the test material or reference material measured in t time.
Compute: